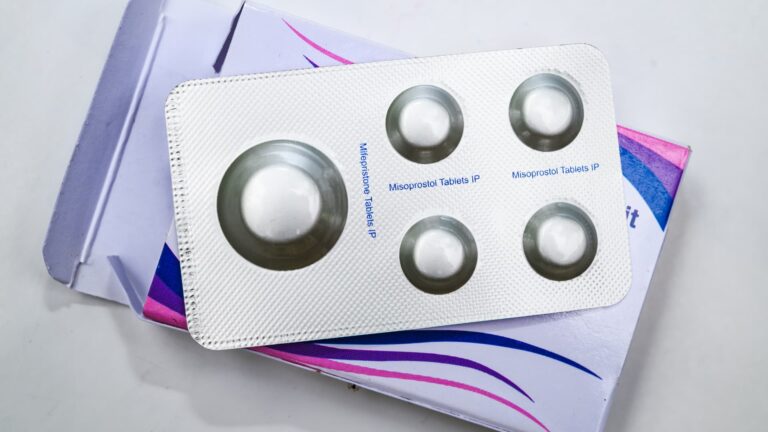
Mifepristone, also referred to as RU-486, is a drug usually utilized in mixture with misoprostol to carry out medical abortion throughout being pregnant and to manage early miscarriage.
Soumyabrata Roy | Noor Pictures | Getty Pictures
WASHINGTON — The Supreme Courtroom on Thursday rejected a problem to the abortion drug mifepristone, which means the generally used drug stays extensively out there.
A courtroom has unanimously held that an anti-abortion physician group difficult the Meals and Drug Administration’s choice to make contraception tablets extra accessible doesn’t have authorized standing to sue. Accordingly, the swimsuit is dismissed.
By dismissing the case on this foundation, the courtroom averted ruling on the authorized foundation for the FDA to carry numerous restrictions, together with these making medicine out there by mail, which means the identical situation is prone to come up in 2019 Return to courtroom within the 12 months.
One other regulatory choice means ladies can nonetheless take the tablet as much as 10 weeks into being pregnant as a substitute of seven.
Likewise, the choice to permit well being care suppliers aside from docs to dispense the tablets will stay in impact.
The ruling comes two years after the courtroom’s 6-3 conservative majority overturned Roe v. Wade, the landmark abortion-rights ruling wherein conservatives States have triggered a brand new wave of abortion restrictions.
The courtroom then stated it was withdrawing from the political debate over abortion, however as litigation over abortion continues to rage, the justices proceed to play a key position.
The mifepristone dispute shouldn’t be the one abortion case at the moment earlier than the courts. It would additionally determine whether or not Idaho’s strict abortion ban prevents emergency room docs from performing abortions when pregnant ladies face harmful problems.
Mifepristone is a part of a two-drug routine accepted by the FDA and is at the moment the commonest type of abortion in the USA.
Actually, 14 states have outright abortion bans, based on the Guttmacher Institute, a analysis group that helps abortion rights.
The FDA has the assist of the pharmaceutical business, which warns that any second-guessing of the approval course of by untrained federal judges may result in confusion and hinder innovation.
The authorized problem was introduced by docs and different medical professionals represented by the conservative Christian authorized group Alliance Defending Freedom.
Final 12 months, U.S. District Decide Matthew Kacsmaryk in Texas issued a sweeping ruling that utterly invalidated the FDA’s approval of the drug, main abortion rights activists to specific doubts that the drug could be out there in The nation was banned from panicking.
Final April, the Supreme Courtroom put that ruling on maintain, which means the drug may stay extensively out there whereas the lawsuit continues.
In August, the fifth U.S. Circuit Courtroom of Appeals in New Orleans narrowed Kacsmaryk’s ruling however preserved his conclusion that the FDA’s transfer to carry restrictions beginning in 2016 was illegal.
Either side appealed to the Supreme Courtroom. The courtroom in December accepted the Biden administration’s enchantment defending the FDA’s later choice, however selected to not hear a problem to mifepristone’s unique approval in 2000.
The Supreme Courtroom centered solely on the FDA’s subsequent actions, together with its preliminary 2021 choice finalized final 12 months to make the drug out there by mail.